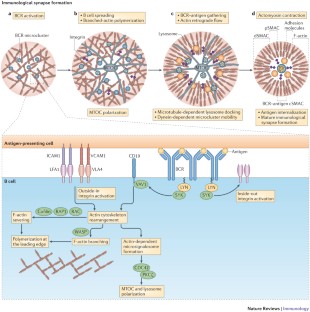
How B cells capture, process and present antigens: a crucial role for cell polarity
B cells are key components of the adaptive immune response. Their differentiation into either specific memory B cells or antibody-secreting plasma cells is a consequence of activation steps that involve the processing and presentation of antigens. The engagement of B cell receptors by surface-tethered antigens leads to the formation of an immunological synapse that coordinates cell signalling events and that promotes antigen uptake for presentation on MHC class II molecules. In this Review, we discuss membrane trafficking and the associated molecular mechanisms that are involved in antigen extraction and processing at the B cell synapse, and we highlight how B cells use cell polarity to coordinate the complex events that ultimately lead to efficient humoral responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Surface phenotypes of naive and memory B cells in mouse and human tissues
Article 22 December 2021
Synergy between B cell receptor/antigen uptake and MHCII peptide editing relies on HLA-DO tuning
Article Open access 25 September 2019
Homeostasis and regulation of autoreactive B cells
Article 07 May 2020
References
- Mitchison, N. A. T-cell–B-cell cooperation. Nature Rev. Immunol.4, 308–312 (2004). ArticleCASGoogle Scholar
- Batista, F. D., Iber, D. & Neuberger, M. S. B cells acquire antigen from target cells after synapse formation. Nature411, 489–494 (2001). ArticleCASPubMedGoogle Scholar
- Bertrand, F. E. et al. Microenvironmental influences on human B-cell development. Immunol. Rev.175, 175–186 (2000). ArticleCASPubMedGoogle Scholar
- von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nature Rev. Immunol.3, 867–878 (2003). ArticleCASGoogle Scholar
- Cyster, J. G. B cell follicles and antigen encounters of the third kind. Nature Immunol.11, 989–996 (2010). ArticleCASGoogle Scholar
- Carrasco, Y. R. & Batista, F. D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity27, 160–171 (2007). ArticleCASPubMedGoogle Scholar
- Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature450, 110–114 (2007). References 6 and 7 highlight roles for subcapsular macrophages in the lymph nodes in the presentation of large antigens to B cellsin vivo. ArticleCASPubMedGoogle Scholar
- Suzuki, K., Grigorova, I., Phan, T. G., Kelly, L. M. & Cyster, J. G. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J. Exp. Med.206, 1485–1493 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Qi, H., Egen, J. G., Huang, A. Y. & Germain, R. N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science312, 1672–1676 (2006). References 8 and 9use two-photon microscopy to visualizein vivohow B cells capture antigen from FDCs in primary follicles or from DCs upon entry to the lymph nodes.ArticleCASPubMedGoogle Scholar
- Pape, K. A., Catron, D. M., Itano, A. A. & Jenkins, M. K. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity26, 491–502 (2007). ArticleCASPubMedGoogle Scholar
- Clark, S. L. Jr. The reticulum of lymph nodes in mice studied with the electron microscope. Am. J. Anat.110, 217–257 (1962). ArticlePubMedGoogle Scholar
- Gretz, J. E., Norbury, C. C., Anderson, A. O., Proudfoot, A. E. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med.192, 1425–1440 (2000). ArticleCASPubMedPubMed CentralGoogle Scholar
- Batista, F. D. & Harwood, N. E. The who, how and where of antigen presentation to B cells. Nature Rev. Immunol.9, 15–27 (2009). ArticleCASGoogle Scholar
- Saez de Guinoa, J., Barrio, L., Mellado, M. & Carrasco, Y. R. CXCL13/CXCR5 signaling enhances BCR-triggered B-cell activation by shaping cell dynamics. Blood118, 1560–1569 (2011). ArticleCASPubMedGoogle Scholar
- Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science285, 221–227 (1999). ArticleCASPubMedGoogle Scholar
- Yuseff, M. I., Lankar, D. & Lennon-Dumenil, A. M. Dynamics of membrane trafficking downstream of B and T cell receptor engagement: impact on immune synapses. Traffic10, 629–636 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Carrasco, Y. R., Fleire, S. J., Cameron, T., Dustin, M. L. & Batista, F. D. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity20, 589–599 (2004). ArticleCASPubMedGoogle Scholar
- Cambier, J. C., Pleiman, C. M. & Clark, M. R. Signal transduction by the B cell antigen receptor and its coreceptors. Annu. Rev. Immunol.12, 457–486 (1994). ArticleCASPubMedGoogle Scholar
- Reth, M. & Wienands, J. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol.15, 453–479 (1997). ArticleCASPubMedGoogle Scholar
- Baba, Y. & Kurosaki, T. Impact of Ca 2+ signaling on B cell function. Trends Immunol.32, 589–594 (2011). ArticleCASPubMedGoogle Scholar
- Fleire, S. J. et al. B cell ligand discrimination through a spreading and contraction response. Science312, 738–741 (2006). This paper describes the two-phase spreading and contraction response exerted by B cells that are in contact with membrane-bound antigens. This response promotes antigen gathering into a central cluster at the synapse.ArticleCASPubMedGoogle Scholar
- Harwood, N. E. & Batista, F. D. Early events in B cell activation. Annu. Rev. Immunol.28, 185–210 (2010). ArticleCASPubMedGoogle Scholar
- Depoil, D. et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nature Immunol.9, 63–72 (2008). ArticleCASGoogle Scholar
- Depoil, D. et al. Early events of B cell activation by antigen. Sci. Signal.2, Pt1 (2009). ArticlePubMedGoogle Scholar
- Tolar, P., Sohn, H. W. & Pierce, S. K. Viewing the antigen-induced initiation of B-cell activation in living cells. Immunol. Rev.221, 64–76 (2008). This review highlights experiments using live-cell imaging technologies that can be used to show the early spatiotemporal signalling events that occur downstream of BCR engagement.ArticleCASPubMedGoogle Scholar
- Treanor, B., Depoil, D., Bruckbauer, A. & Batista, F. D. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J. Exp. Med.208, 1055–1068 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Schnyder, T. et al. B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity34, 905–918 (2011). ArticleCASPubMedGoogle Scholar
- Arana, E. et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity28, 88–99 (2008). ArticleCASPubMedGoogle Scholar
- Lin, K. B. et al. The rap GTPases regulate B cell morphology, immune-synapse formation, and signaling by particulate B cell receptor ligands. Immunity28, 75–87 (2008). ArticleCASPubMedGoogle Scholar
- Freeman, S. A. et al. Cofilin-mediated F-actin severing is regulated by the Rap GTPase and controls the cytoskeletal dynamics that drive lymphocyte spreading and BCR microcluster formation. J. Immunol.187, 5887–5900 (2011). ArticleCASPubMedGoogle Scholar
- Hao, S. & August, A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol. Biol. Cell16, 2275–2284 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Treanor, B. et al. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity32, 187–199 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Meyer-Bahlburg, A. et al. Wiskott–Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis. Blood112, 4158–4169 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tsourkas, P. K., Baumgarth, N., Simon, S. I. & Raychaudhuri, S. Mechanisms of B-cell synapse formation predicted by Monte Carlo simulation. Biophys. J.92, 4196–4208 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tsourkas, P. K., Longo, M. L. & Raychaudhuri, S. Monte Carlo study of single molecule diffusion can elucidate the mechanism of B cell synapse formation. Biophys. J.95, 1118–1125 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tsourkas, P. K. & Raychaudhuri, S. Modeling of B cell synapse formation by Monte Carlo simulation shows that directed transport of receptor molecules is a potential formation mechanism. Cell. Mol. Bioeng.3, 256–268 (2010). ArticleCASPubMedGoogle Scholar
- Stinchcombe, J. C. et al. Centriole polarisation to the immunological synapse directs secretion from cytolytic cells of both the innate and adaptive immune systems. BMC Biol.9, 45 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
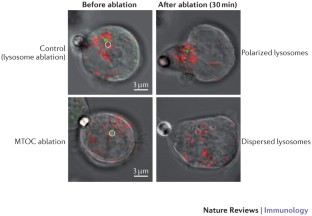
- Yuseff, M. I. et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity35, 361–374 (2011). This paper was the first to show how B cells use conserved polarity mechanisms to locally recruit and secrete lysosomes at the immunological synapsein order to extract and present immobilized antigens on MHC class II molecules.ArticleCASPubMedGoogle Scholar
- Combs, J. et al. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl Acad. Sci. USA103, 14883–14888 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gomes, E. R., Jani, S. & Gundersen, G. G. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell121, 451–463 (2005). ArticleCASPubMedGoogle Scholar
- Iden, S. & Collard, J. G. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature Rev. Mol. Cell Biol.9, 846–859 (2008). ArticleCASGoogle Scholar
- Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity15, 751–761 (2001). ArticleCASPubMedGoogle Scholar
- Poo, W. J., Conrad, L. & Janeway, C. A. Jr. Receptor-directed focusing of lymphokine release by helper T cells. Nature332, 378–380 (1988). ArticleCASPubMedGoogle Scholar
- Stinchcombe, J. C., Majorovits, E., Bossi, G., Fuller, S. & Griffiths, G. M. Centrosome polarization delivers secretory granules to the immunological synapse. Nature443, 462–465 (2006). ArticleCASPubMedGoogle Scholar
- Chemin, K. et al. Cytokine secretion by CD4 + T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J. Immunol.189, 2159–2168 (2012). ArticleCASPubMedGoogle Scholar
- Xu, J. et al. Mechanism of polarized lysosome exocytosis in epithelial cells. J. Cell Sci.125, 5937–5943 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Jordens, I., Marsman, M., Kuijl, C. & Neefjes, J. Rab proteins, connecting transport and vesicle fusion. Traffic6, 1070–1077 (2005). ArticleCASPubMedGoogle Scholar
- Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell92, 759–772 (1998). ArticleCASPubMedGoogle Scholar
- Das, V. et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity20, 577–588 (2004). ArticleCASPubMedGoogle Scholar
- Andrews, N. W. Regulated secretion of conventional lysosomes. Trends Cell Biol.10, 316–321 (2000). ArticleCASPubMedGoogle Scholar
- Stinchcombe, J. C. et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol.152, 825–834 (2001). ArticleCASPubMedPubMed CentralGoogle Scholar
- Holt, O. et al. Slp1 and Slp2-a localize to the plasma membrane of CTL and contribute to secretion from the immunological synapse. Traffic9, 446–457 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Haka, A. S. et al. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol. Biol. Cell20, 4932–4940 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Aucher, A., Magdeleine, E., Joly, E. & Hudrisier, D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood111, 5621–5628 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Joly, E. & Hudrisier, D. What is trogocytosis and what is its purpose? Nature Immunol.4, 815 (2003). ArticleCASGoogle Scholar
- Arndt, S. O. et al. Functional HLA-DM on the surface of B cells and immature dendritic cells. EMBO J.19, 1241–1251 (2000). ArticleCASPubMedPubMed CentralGoogle Scholar
- Santambrogio, L. et al. Extracellular antigen processing and presentation by immature dendritic cells. Proc. Natl Acad. Sci. USA96, 15056–15061 (1999). ArticleCASPubMedPubMed CentralGoogle Scholar
- Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science307, 1630–1634 (2005). ArticleCASPubMedGoogle Scholar
- Lennon-Dumenil, A. M. et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med.196, 529–540 (2002). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lankar, D. et al. Dynamics of major histocompatibility complex class II compartments during B cell receptor-mediated cell activation. J. Exp. Med.195, 461–472 (2002). ArticleCASPubMedPubMed CentralGoogle Scholar
- Stoddart, A. et al. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity17, 451–462 (2002). ArticleCASPubMedGoogle Scholar
- Zhang, M. et al. Ubiquitinylation of Igβ dictates the endocytic fate of the B cell antigen receptor. J. Immunol.179, 4435–4443 (2007). ArticleCASPubMedGoogle Scholar
- Drake, L., McGovern-Brindisi, E. M. & Drake, J. R. BCR ubiquitination controls BCR-mediated antigen processing and presentation. Blood108, 4086–4093 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Katkere, B., Rosa, S. & Drake, J. R. The Syk-binding ubiquitin ligase c-Cbl mediates signaling-dependent B cell receptor ubiquitination and B cell receptor-mediated antigen processing and presentation. J. Biol. Chem.287, 16636–16644 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kim, Y. M. et al. Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc. Natl Acad. Sci. USA103, 3327–3332 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chaturvedi, A., Martz, R., Dorward, D., Waisberg, M. & Pierce, S. K. Endocytosed BCRs sequentially regulate MAPK and Akt signaling pathways from intracellular compartments. Nature Immunol.12, 1119–1126 (2011). ArticleCASGoogle Scholar
- Chatterjee, P., Tiwari, R. K., Rath, S., Bal, V. & George, A. Modulation of antigen presentation and B cell receptor signaling in B cells of beige mice. J. Immunol.188, 2695–2702 (2012). This paper shows that BCR signalling that is initiated at the plasma membrane continues in intracellular compartments, initiating a sequential phosphorylation of kinases.ArticleCASPubMedGoogle Scholar
- Onabajo, O. O. et al. Actin-binding protein 1 regulates B cell receptor-mediated antigen processing and presentation in response to B cell receptor activation. J. Immunol.180, 6685–6695 (2008). ArticleCASPubMedGoogle Scholar
- Sharma, S., Orlowski, G. & Song, W. Btk regulates B cell receptor-mediated antigen processing and presentation by controlling actin cytoskeleton dynamics in B cells. J. Immunol.182, 329–339 (2009). ArticleCASPubMedGoogle Scholar
- Le Roux, D. et al. Syk-dependent actin dynamics regulate endocytic trafficking and processing of antigens internalized through the B-cell receptor. Mol. Biol. Cell18, 3451–3462 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, Q. et al. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J. Cell Biol.160, 565–575 (2003). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hornstein, I., Alcover, A. & Katzav, S. Vav proteins, masters of the world of cytoskeleton organization. Cell. Signal.16, 1–11 (2004). ArticleCASPubMedGoogle Scholar
- Weber, M. et al. Phospholipase C-γ2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J. Exp. Med.205, 853–868 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gondre-Lewis, T. A., Moquin, A. E. & Drake, J. R. Prolonged antigen persistence within nonterminal late endocytic compartments of antigen-specific B lymphocytes. J. Immunol.166, 6657–6664 (2001). ArticleCASPubMedGoogle Scholar
- Vascotto, F. et al. The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J. Cell Biol.176, 1007–1019 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Pereira, J. P., Kelly, L. M. & Cyster, J. G. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int. Immunol.22, 413–419 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature416, 94–99 (2002). ArticlePubMedGoogle Scholar
- Pereira, J. P., Kelly, L. M., Xu, Y. & Cyster, J. G. EBI2 mediates B cell segregation between the outer and centre follicle. Nature460, 1122–1126 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Duchez, S., Rodrigues, M., Bertrand, F. & Valitutti, S. Reciprocal polarization of T and B cells at the immunological synapse. J. Immunol.187, 4571–4580 (2011). ArticleCASPubMedGoogle Scholar
- Cunningham, A. F. et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol.178, 6200–6207 (2007). ArticleCASPubMedGoogle Scholar
- Allen, C. D., Okada, T. & Cyster, J. G. Germinal-center organization and cellular dynamics. Immunity27, 190–202 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- MacLennan, I. C. Germinal centers. Annu. Rev. Immunol.12, 117–139 (1994). ArticleCASPubMedGoogle Scholar
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol.29, 621–663 (2011). ArticleCASPubMedGoogle Scholar
- Drubin, D. G. & Nelson, W. J. Origins of cell polarity. Cell84, 335–344 (1996). ArticleCASPubMedGoogle Scholar
- Guo, F., Velu, C. S., Grimes, H. L. & Zheng, Y. Rho GTPase Cdc42 is essential for B-lymphocyte development and activation. Blood114, 2909–2916 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Martin, P. et al. Role of ζ-PKC in B-cell signaling and function. EMBO J.21, 4049–4057 (2002). ArticleCASPubMedPubMed CentralGoogle Scholar
- Thaunat, O. et al. Asymmetric segregation of polarized antigen on B cell division shapes presentation capacity. Science335, 475–479 (2012). ArticleCASPubMedGoogle Scholar
- Shlomchik, M. J. & Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev.247, 52–63 (2012). ArticlePubMedGoogle Scholar
- Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol.30, 429–457 (2012). ArticleCASPubMedGoogle Scholar
- Victora, G. D. et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell143, 592–605 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Randall, K. L. et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nature Immunol.10, 1283–1291 (2009). This work shows that DOCK8 mutations impair the accumulation of the integrin ligand ICAM1 in the B cell immunological synapse. This leads to B cell-deficient signalling and highlights how humoral immunodeficiency is dependent on the organization of the B cell immunological synapse.ArticleCASGoogle Scholar
- Harada, Y. et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood119, 4451–4461 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hauser, A. E. et al. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity26, 655–667 (2007). Using multiphotonin vivomicroscopy, this work describes the migration patterns of B cells between the dark and light zones of the germinal centre, detailing the morphological changes that B cells undergo during this movement.ArticleCASPubMedGoogle Scholar
- Barnett, B. E. et al. Asymmetric B cell division in the germinal center reaction. Science335, 342–344 (2012). This study describes how, following cell division, germinal centre B cells asymmetrically segregate molecules that are involved in B cell fate, as well as the ancestral polarity protein PKCζ,to generate unequal daughter cells.ArticleCASPubMedGoogle Scholar
- Li, R. & Bowerman, B. Symmetry breaking in biology. Cold Spring Harb. Perspect. Biol.2, a003475 (2010). ArticlePubMedPubMed CentralCASGoogle Scholar
- Mogilner, A., Allard, J. & Wollman, R. Cell polarity: quantitative modeling as a tool in cell biology. Science336, 175–179 (2012). ArticleCASPubMedGoogle Scholar
- Hat, B., Kazmierczak, B. & Lipniacki, T. B cell activation triggered by the formation of the small receptor cluster: a computational study. PLoS Comput. Biol.7, e1002197 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tsourkas, P. K., Liu, W., Das, S. C., Pierce, S. K. & Raychaudhuri, S. Discrimination of membrane antigen affinity by B cells requires dominance of kinetic proofreading over serial engagement. Cell. Mol. Immunol.9, 62–74 (2011). ArticlePubMedPubMed CentralCASGoogle Scholar
- Goehring, N. W. et al. Polarization of PAR proteins by advective triggering of a pattern-forming system. Science334, 1137–1141 (2011). ArticleCASPubMedGoogle Scholar
- Wan, Z. et al. B cell activation is regulated by the stiffness properties of the substrate presenting the antigens. J. Immunol.190, 4661–4675 (2013). ArticleCASPubMedGoogle Scholar
- Wedlich-Soldner, R., Altschuler, S., Wu, L. & Li, R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science299, 1231–1235 (2003). This is an important paper, showing the role of CDC42 in cell polarity. In this paper, experiments that are corroborated by a mathematical model show that a stable axis of polarity arises in yeast from a positive feedback mechanism that amplifies a random concentration of activated CDC42. This work suggests that spontaneous polarization might occur and could be stabilized by external cues.ArticleCASPubMedGoogle Scholar
- Gierer, A. & Meinhardt, H. A theory of biological pattern formation. Kybernetik12, 30–39 (1972). ArticleCASPubMedGoogle Scholar
- Jilkine, A. & Edelstein-Keshet, L. A comparison of mathematical models for polarization of single eukaryotic cells in response to guided cues. PLoS Comput. Biol.7, e1001121 (2011). This paper is a detailed survey of the different models of cell polarity. The authors identify the general features of a polarizing cell that are common to all systems, they highlight the advantages and the disadvantages of different mathematical models and they test the response of the different models to a given stimulus.ArticleCASPubMedPubMed CentralGoogle Scholar
- Baracho, G. V., Miletic, A. V., Omori, S. A., Cato, M. H. & Rickert, R. C. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr. Opin. Immunol.23, 178–183 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Liu, C. et al. A balance of Bruton's tyrosine kinase and SHIP activation regulates B cell receptor cluster formation by controlling actin remodeling. J. Immunol.187, 230–239 (2011). ArticleCASPubMedGoogle Scholar
- Houk, A. R. et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell148, 175–188 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wedlich-Soldner, R. & Li, R. Spontaneous cell polarization: undermining determinism. Nature Cell Biol.5, 267–270 (2003). ArticleCASPubMedGoogle Scholar
- Mattila, P. K. et al. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity38, 461–474 (2013). ArticleCASPubMedGoogle Scholar
- Cheng, P. C., Steele, C. R., Gu, L., Song, W. & Pierce, S. K. MHC class II antigen processing in B cells: accelerated intracellular targeting of antigens. J. Immunol.162, 7171–7180 (1999). CASPubMedGoogle Scholar
- Forquet, F. et al. Presentation of antigens internalized through the B cell receptor requires newly synthesized MHC class II molecules. J. Immunol.162, 3408–3416 (1999). CASPubMedGoogle Scholar
- Wolf, P. R. & Ploegh, H. L. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu. Rev. Cell Dev. Biol.11, 267–306 (1995). ArticleCASPubMedGoogle Scholar
- Villadangos, J. A. et al. Proteases involved in MHC class II antigen presentation. Immunol. Rev.172, 109–120 (1999). ArticleCASPubMedGoogle Scholar
- Watts, C. Antigen processing in the endocytic compartment. Curr. Opin. Immunol.13, 26–31 (2001). ArticleCASPubMedGoogle Scholar
- Brezski, R. J. & Monroe, J. G. B cell antigen receptor-induced Rac1 activation and Rac1-dependent spreading are impaired in transitional immature B cells due to levels of membrane cholesterol. J. Immunol.179, 4464–4472 (2007). ArticleCASPubMedGoogle Scholar
- Walmsley, M. J. et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science302, 459–462 (2003). ArticleCASPubMedGoogle Scholar
- Westerberg, L. S. et al. Wiskott–Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B-cell development and function. Blood119, 3966–3974 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
Acknowledgements
The authors kindly thank C. Hivroz, Y. R. Carrasco, D. Obino and D. Lankar for discussions and critical reading of the manuscript. This work was funded by grants from the European Research Council to A.-M.L.-D. and the L'Agence nationale de la recherche Jeunes Chercheuses et Jeunes Chercheurs programme to P.P.
Author information
Authors and Affiliations
- Inserm U932, Institut Curie, 12 rue Lhomond, Paris, 75005, France Maria-Isabel Yuseff, Paolo Pierobon, Anne Reversat & Ana-Maria Lennon-Duménil







